26th June 2021
Different chemical mechanisms in glutamatergic synapses support different types of memory
Different chemical mechanisms in glutamatergic synapses support different types of memory
Five different types of memory are observed in the brain: working, priming, semantic, episodic, and procedural. Working memory is the ability to hold information active in the brain for a few seconds. Priming is the ability to retain perceptual information active for a minute or two, even though the perception was not conscious. Semantic memory is the ability to recall facts and words for periods ranging from hours to very long term. Episodic memory is the ability to recall information about individual events, again for periods ranging from hours to very long term. Procedural memory is the ability to carry out skills learned in the past. Procedural memories are retained for long periods.
MEMORY INVOLVES INDIRECT ACTIVATION OF NEURONS
Each type of memory involves accessing information about the past. Such access means that some of the neurons active during past experiences must be reactivated, in the absence of the sensory or other inputs that drove their activation during the original experiences. Neurons do not “know” anything about facts, events or skills. The only type of information available to neurons is the simultaneous activity of groups of other neurons. Such activity information must therefore be used to implement the various types of memory. If a group of currently active neurons was also active in the past at the same time as some group of currently inactive neurons, the active group can indirectly activate the inactive group on the basis of that past temporally correlated activity. Such indirect activations can access information about the past and are the basis for memory.
Priming memory depends on the indirect activation of neurons on the basis of recent simultaneous activity. Semantic memory depends on indirect activation of neurons on the basis of frequent past simultaneous activity by the two groups. Memory for events depends on indirect activation on the basis of past simultaneous activity during a period in which there were many changes to neuron receptive fields. Working memory maintains the activity of neurons on the basis of the current simultaneous activity of a group of neurons. Procedural memory activates neurons on the basis of past simultaneous activity when other neurons correlating with the presence of a reward were active.
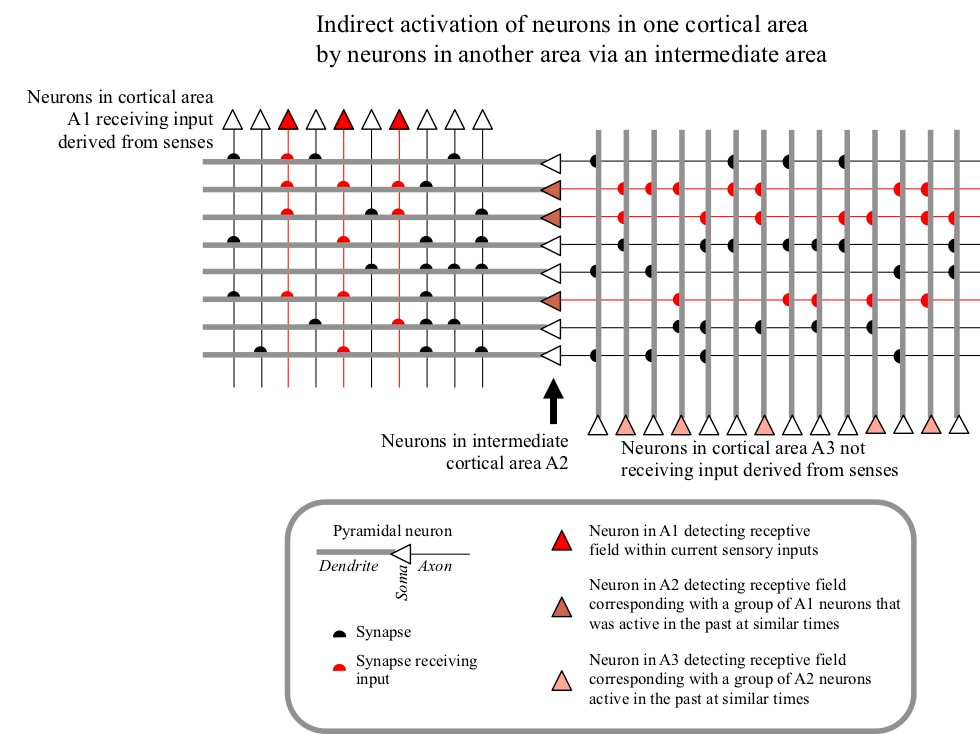
GROUPS OF NEURONS INDIRECTLY ACTIVATE OTHER GROUPS VIA INTERMEDIATE NEURONS
These indirect activations are generally carried out via intermediate neurons. Such an intermediate neuron has inputs from a group of neurons that has been active in the past at similar times. The intermediate neuron targets a second group of neurons that has some kind of temporally correlated past activity with the first group. Activity of a significant proportion of neurons in the first group may therefore drive indirect activation of the neurons in the second group. In other words, these intermediate neurons have receptive fields corresponding with groups of other neurons active at a similar time in the past, and target neurons that were active when they themselves were active.
MEMORY INVOLVES INDIRECT ACTIVATION OF NEURONS
Each type of memory involves accessing information about the past. Such access means that some of the neurons active during past experiences must be reactivated, in the absence of the sensory or other inputs that drove their activation during the original experiences. Neurons do not “know” anything about facts, events or skills. The only type of information available to neurons is the simultaneous activity of groups of other neurons. Such activity information must therefore be used to implement the various types of memory. If a group of currently active neurons was also active in the past at the same time as some group of currently inactive neurons, the active group can indirectly activate the inactive group on the basis of that past temporally correlated activity. Such indirect activations can access information about the past and are the basis for memory.
Priming memory depends on the indirect activation of neurons on the basis of recent simultaneous activity. Semantic memory depends on indirect activation of neurons on the basis of frequent past simultaneous activity by the two groups. Memory for events depends on indirect activation on the basis of past simultaneous activity during a period in which there were many changes to neuron receptive fields. Working memory maintains the activity of neurons on the basis of the current simultaneous activity of a group of neurons. Procedural memory activates neurons on the basis of past simultaneous activity when other neurons correlating with the presence of a reward were active.
GROUPS OF NEURONS INDIRECTLY ACTIVATE OTHER GROUPS VIA INTERMEDIATE NEURONS
These indirect activations are generally carried out via intermediate neurons. Such an intermediate neuron has inputs from a group of neurons that has been active in the past at similar times. The intermediate neuron targets a second group of neurons that has some kind of temporally correlated past activity with the first group. Activity of a significant proportion of neurons in the first group may therefore drive indirect activation of the neurons in the second group. In other words, these intermediate neurons have receptive fields corresponding with groups of other neurons active at a similar time in the past, and target neurons that were active when they themselves were active.
To support the different types of memory, the receptive fields of these intermediate neurons must last for different periods of time. Neuron receptive fields lasting only a few seconds are needed to support working memory. Receptive fields lasting minutes are required to support priming. Semantic, episodic and procedural memories can last for from hours and days up to many years.
Ultimately, receptive fields are defined by the weights of synapses between neurons. In the case of working, priming, semantic and episodic memory the key synapses are between cortical pyamidal neurons. In the case of procedural memory, the key synapses are made by cortical pyramidal neurons on to medium spiny neurons in the striatum of the basal ganglia. All these synapses use the neurotransmitter glutamate that is synthesized in pyramidal neurons, and the synapses are therefore called glutamatergic. Chemical mechanisms that change the weights of glutamatergic synapses on different timescales therefore support different memory types.
GENERAL PROPERTIES OF NEURONS
Most human cells are compact bodies completely surrounded by a membrane. Neurons also have a compact body, called the soma, but differ from most other cells in having a number of extensions. These extensions bulge out from the soma for millimetres or even centimetres, but are completely inside the membrane. One extension is called the axon, it branches extensively to carry outputs from the neuron to target neurons. These outputs reach the target neurons at connection points called synapses. Neurons also have multiple extensions called dendrites, that generally branch to a smaller degree than the axon. Most synapses are located on the dendrites.
The membrane potential and ion channels
Chemical processes pump sodium (Na+), calcium (Ca++) and chloride (Cl–) ions from the inside to the outside of the neuron. Potassium (K+) and bicarbonate (HCO3–) ions are pumped into the neuron. The resultant ion concentration differences maintain an electrical voltage difference of 70 millivolts between the outside and the inside of all points on the membrane, with the inside negative. This voltage difference is called the resting potential.
Although the membrane itself is impermeable to ions, it is penetrated by large numbers of chemicals called ion channels that are generally closed but under some circumstances will open and allow ions to pass through the membrane. One ion channel will allow one type of ion to pass from the region of high concentration to the region of low concentration. Such a flow can change the membrane potential in the vicinity of the channel away from its resting value. For example, if a sodium channel opens, positive charge flows in and makes the local membrane potential less negative. If a potassium channel opens, positive charge flows out and makes the membrane potential more negative. If a calcium channel opens, calcium ions flow in, making the membrane potential less negative but in addition the ions trigger cascades of chemical processes within the neuron.
There are two mechanisms by which ion channels are opened. One is that a chemical called a neurotransmitter located outside the neuron binds to the ion channel. This binding only lasts for a short while, but while bound the ion channel is open. Ion channels of this type are called neurotransmitter gated. The second mechanism is that a channel opens if the membrane potential shifts away from the resting potential into a voltage range specific to the channel. Such ion channels are called voltage gated. A few types of ion channels require both binding by a neurotransmitter and a change in membrane potential.
G-protein receptors
There are additional chemicals spanning the membrane called G-protein receptors. These receptors also bind to neurotransmitter molecules, but when bound they initiate cascades of chemical processes within the neuron.
THE GLUTAMATERGIC SYNAPSE
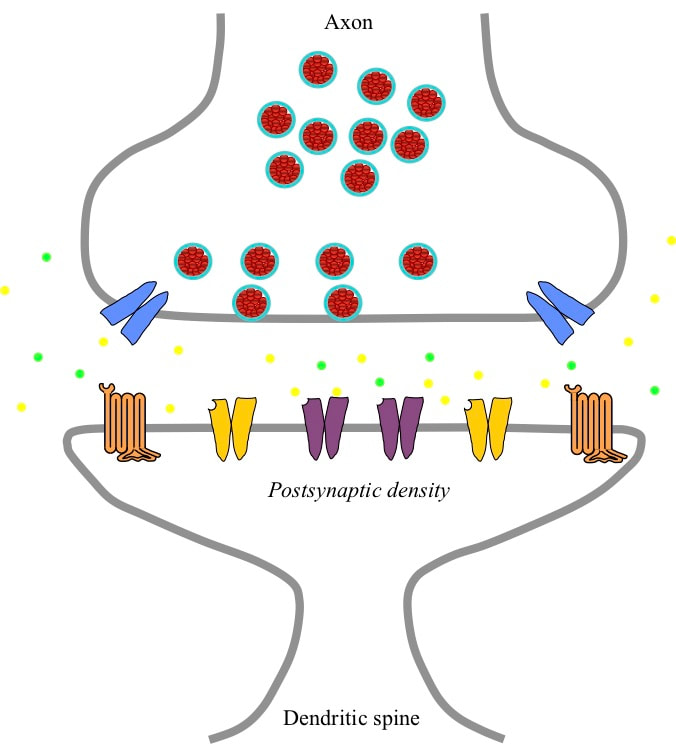
The signals indicating receptive field detections by a pyramidal neuron are 100 millivolt electrical spikes called action potentials. These action potentials are launched into the axon of the neuron. That axon branches to carry the signal to glutamatergic synapses on large numbers of different target neurons. A glutamatergic synapse has two parts separated by a narrow gap called the synaptic cleft. The axonic part is a swelling at the tip of the incoming axon branch. Opposite the axon the neuron forms a spine that sticks out from the dendrite, and the dendritic part of the synapse is a swelling at the tip of the spine. The spines isolate the dendritic parts of different synapses from each other, making it possible to change synaptic weights independently.
Ultimately, receptive fields are defined by the weights of synapses between neurons. In the case of working, priming, semantic and episodic memory the key synapses are between cortical pyamidal neurons. In the case of procedural memory, the key synapses are made by cortical pyramidal neurons on to medium spiny neurons in the striatum of the basal ganglia. All these synapses use the neurotransmitter glutamate that is synthesized in pyramidal neurons, and the synapses are therefore called glutamatergic. Chemical mechanisms that change the weights of glutamatergic synapses on different timescales therefore support different memory types.
GENERAL PROPERTIES OF NEURONS
Most human cells are compact bodies completely surrounded by a membrane. Neurons also have a compact body, called the soma, but differ from most other cells in having a number of extensions. These extensions bulge out from the soma for millimetres or even centimetres, but are completely inside the membrane. One extension is called the axon, it branches extensively to carry outputs from the neuron to target neurons. These outputs reach the target neurons at connection points called synapses. Neurons also have multiple extensions called dendrites, that generally branch to a smaller degree than the axon. Most synapses are located on the dendrites.
The membrane potential and ion channels
Chemical processes pump sodium (Na+), calcium (Ca++) and chloride (Cl–) ions from the inside to the outside of the neuron. Potassium (K+) and bicarbonate (HCO3–) ions are pumped into the neuron. The resultant ion concentration differences maintain an electrical voltage difference of 70 millivolts between the outside and the inside of all points on the membrane, with the inside negative. This voltage difference is called the resting potential.
Although the membrane itself is impermeable to ions, it is penetrated by large numbers of chemicals called ion channels that are generally closed but under some circumstances will open and allow ions to pass through the membrane. One ion channel will allow one type of ion to pass from the region of high concentration to the region of low concentration. Such a flow can change the membrane potential in the vicinity of the channel away from its resting value. For example, if a sodium channel opens, positive charge flows in and makes the local membrane potential less negative. If a potassium channel opens, positive charge flows out and makes the membrane potential more negative. If a calcium channel opens, calcium ions flow in, making the membrane potential less negative but in addition the ions trigger cascades of chemical processes within the neuron.
There are two mechanisms by which ion channels are opened. One is that a chemical called a neurotransmitter located outside the neuron binds to the ion channel. This binding only lasts for a short while, but while bound the ion channel is open. Ion channels of this type are called neurotransmitter gated. The second mechanism is that a channel opens if the membrane potential shifts away from the resting potential into a voltage range specific to the channel. Such ion channels are called voltage gated. A few types of ion channels require both binding by a neurotransmitter and a change in membrane potential.
G-protein receptors
There are additional chemicals spanning the membrane called G-protein receptors. These receptors also bind to neurotransmitter molecules, but when bound they initiate cascades of chemical processes within the neuron.
THE GLUTAMATERGIC SYNAPSE
The signals indicating receptive field detections by a pyramidal neuron are 100 millivolt electrical spikes called action potentials. These action potentials are launched into the axon of the neuron. That axon branches to carry the signal to glutamatergic synapses on large numbers of different target neurons. A glutamatergic synapse has two parts separated by a narrow gap called the synaptic cleft. The axonic part is a swelling at the tip of the incoming axon branch. Opposite the axon the neuron forms a spine that sticks out from the dendrite, and the dendritic part of the synapse is a swelling at the tip of the spine. The spines isolate the dendritic parts of different synapses from each other, making it possible to change synaptic weights independently.
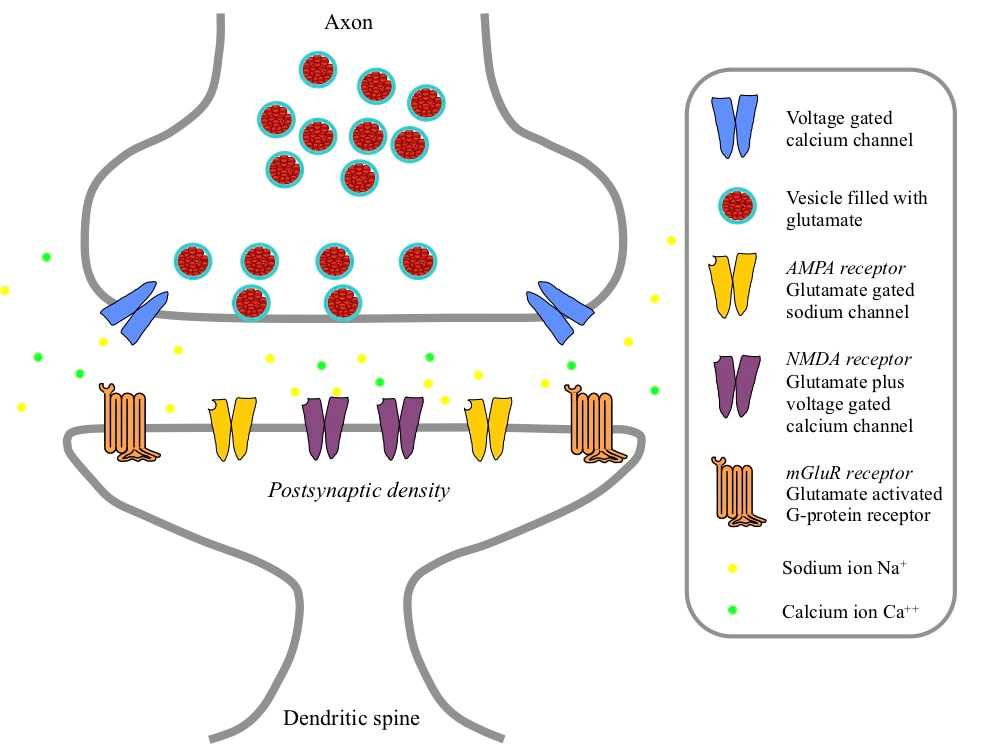
Inside the axonic part of the synapse are a number of vesicles – small vessels surrounded by a membrane and containing glutamate molecules. In the wall of the axonic part are numbers of voltage gated calcium ion channels, which if opened will allow an inflow of calcium ions.
In the dendritic part of the synapse there is what is called a postsynaptic density adjacent to the cleft. This density is made up of a large number of molecules imbedded in the membrane that are receptors for glutamate. If glutamate binds to such a molecule, changes occur that depend on the type of receptor. There are three different types of receptor. In the centre of the postsynaptic density there are NMDA receptors. These are calcium ion channels that open if the membrane potential drops close to zero at a time when the channel is bound by glutamate. In a ring further out in the postsynaptic density there are AMPA ion channels. These are sodium ion channels that open if they are bound by glutamate. Around the periphery of the postsynaptic density there are mGlu receptors (mGluRs). If bound by glutamate these G-protein receptors trigger cascades of chemical reactions within the neuron.
ARRIVAL OF AN ACTION POTENTIAL AT A GLUTAMATERGIC SYNAPSE
When a pyramidal neuron detects its receptive field, it generates an action potential that travels down its axon to glutamatergic synapses on all the targeted neurons. When the action potential arrives at the synapse, it triggers opening of the voltage gated calcium ion channels in the axonic part. These channels allow an influx of calcium ions, which trigger chemical processes leading to the release of the neurotransmitter glutamate from some of the vesicles into the synaptic cleft. In the cleft there are chemical processes that constantly clear away the glutamate, so it is only present for a short while.
The glutamate molecules diffuse across the cleft and bind to the NMDA, AMPA and mGlu receptors in the postsynaptic density. Once bound, the AMPA receptors immediately open and allow sodium ions to pass into the neuron. Binding lasts for a short while and the glutamate is then released and the channel closes again. The short flow of sodium ions is effectively an injection of positive charge, which slightly reduces the membrane potential. The NMDA receptors bind to glutamate, but the slight reduction in membrane potential caused by the AMPA current injection does not move that potential into the range where the NMDA receptors open. However, the central location of the NMDA receptors exposes them to more glutamate, and glutamate remains bound to NMDA receptors for longer. The mGluRs are located further out, and are more likely to be bound by glutamate if several action potentials arrive close together in time.
The reduction in membrane potential resulting from the AMPA current propagates down the spine and into the local dendrite branch. There it can add to the effects of action potentials arriving at other synapses on the same dendritic branch. If the total for the branch is sufficient, it can propagate deeper into the dendrite and add to the effects of other branches. If enough action potentials have recently arrived across the neuron, the neuron will fire and send out its own action potential.
CHANGES TO SYNAPTIC WEIGHTS
The electrical charge injected by an action potential encourages the target neuron to fire. The amount of charge that is injected at a synapse by one action potential is called the synaptic weight. In the case of a synapse between pyramidal neurons, the synaptic weight defines the contribution of the source receptive field to the target receptive field. In the case of a synapse made by a pyramidal neuron on to a medium spiny neuron, the synaptic weight defines the recommendation weight of the receptive field in favour of the behaviour corresponding with the medium spiny neuron. Hence changes to synaptic weights are the mechanisms implementing changes to receptive fields and to recommendation weights. These changes are the detailed mechanisms supporting memory.
The weight of a glutamatergic synapse is defined by a number of factors. One factor is the number of AMPA channels in the synapse. A related factor is the size of the synapse, because a larger synapse has room for more channels. Another factor is the amount of charge that can travle through one AMPA channel during the time it is open. Yet another factor is the proportion of AMPA channels that open, which is determined by the amount of glutamate released by the action potential. All of these factors can be influenced by different mechanisms, thus managing the synaptic weight.
When an action potential arrives at a synapse, calcium ions enter the axonic part through voltage gated calcium channels, trriggering release of glutamate. The calcium ions are removed over a period of seconds. If another action potential arrives within seconds of the first, the new calcium ion influx is reinforced by the ions remaining from the previous action potential, resulting in a largeer release of glutamate. The larger release means that a higher proportion of the AMPA receptors open in the postsynaptic density, increasing the effect of the action potential. Hence there is a temporary increase in synaptic weight that lasts for a few seconds.
After an action potential arrives at a synapse, glutamate binds to NMDA receptors and remains bound for a short while. When a pyramidal neuron fires, it launches an action potential down its axon. In addition, an action potential is launched that backpropagates into the dendritic tree of the neuron. If the backpropagating action potential arrives while glutamate is still bound to the NMDA receptor, the receptor opens and allows calcium ions to enter. An immediate result is a series of chemical reactions that increase the conductance of the AMPA receptors, in other words the amount of sodium ions they allow to pass when open. This increase decays back in a period from minutes to hours, so for that length of time the synaptic weight has increased.
If multiple action potentials arrive close together and the neuron fires, NMDA channels open and in addition sufficient glutamate diffuses to the edge of the postsynaptic density that the mGlu receptors are activated. This combination results in a cascade of chemical processes resulting in the synthesis of more AMPA channels, that are inserted in the postsynaptic density and increase the synaptic weight. However, this increase is temporary, lasting a matter of hours. The decay occurs because although the additional AMPA channels force an expansion in the size of the synapse to accommodate them, the synapse gradually reverts to its original size, squeezing out the extra channels.
If in addition to activation of NMDA and mGlu receptors, before the synapse has decayed back to its regular size a nearby dopamine synapse gets an input, a combination of chemical cascades are activated leading to synthesis of messenger RNA coding for the cytoskeleton molecule actin. This mRNA is transported to the base of the spine, where it drives a higher level of actin synthesis. The additional actin sets the synaptic size larger, resulting in room for more AMPA channels. Synaptic weight increases by this mechanism last much longer term.
MEMORY AND THE TIMESCALE OF SYNAPTIC WEGHT CHANGES
Working memory lasts for a matter of seconds. Definition of the receptive fields that support working memory depend on increased release of glutamate. Priming memory lasts for a matter of minutes, and definition of the receptive fields supporting priming memory depend on increased conductance of AMPA receptors. Semantic and episodic memory last for hours up to very long term. The shorter end of this range depend on creation of extra AMPA channels. Longer term declarative memories depend on stable increases in synaptic size. Procedural memory depends on long term changes to synaptic weights on medium spiny neurons, and also depend on stable changes to synaptic size.
In the dendritic part of the synapse there is what is called a postsynaptic density adjacent to the cleft. This density is made up of a large number of molecules imbedded in the membrane that are receptors for glutamate. If glutamate binds to such a molecule, changes occur that depend on the type of receptor. There are three different types of receptor. In the centre of the postsynaptic density there are NMDA receptors. These are calcium ion channels that open if the membrane potential drops close to zero at a time when the channel is bound by glutamate. In a ring further out in the postsynaptic density there are AMPA ion channels. These are sodium ion channels that open if they are bound by glutamate. Around the periphery of the postsynaptic density there are mGlu receptors (mGluRs). If bound by glutamate these G-protein receptors trigger cascades of chemical reactions within the neuron.
ARRIVAL OF AN ACTION POTENTIAL AT A GLUTAMATERGIC SYNAPSE
When a pyramidal neuron detects its receptive field, it generates an action potential that travels down its axon to glutamatergic synapses on all the targeted neurons. When the action potential arrives at the synapse, it triggers opening of the voltage gated calcium ion channels in the axonic part. These channels allow an influx of calcium ions, which trigger chemical processes leading to the release of the neurotransmitter glutamate from some of the vesicles into the synaptic cleft. In the cleft there are chemical processes that constantly clear away the glutamate, so it is only present for a short while.
The glutamate molecules diffuse across the cleft and bind to the NMDA, AMPA and mGlu receptors in the postsynaptic density. Once bound, the AMPA receptors immediately open and allow sodium ions to pass into the neuron. Binding lasts for a short while and the glutamate is then released and the channel closes again. The short flow of sodium ions is effectively an injection of positive charge, which slightly reduces the membrane potential. The NMDA receptors bind to glutamate, but the slight reduction in membrane potential caused by the AMPA current injection does not move that potential into the range where the NMDA receptors open. However, the central location of the NMDA receptors exposes them to more glutamate, and glutamate remains bound to NMDA receptors for longer. The mGluRs are located further out, and are more likely to be bound by glutamate if several action potentials arrive close together in time.
The reduction in membrane potential resulting from the AMPA current propagates down the spine and into the local dendrite branch. There it can add to the effects of action potentials arriving at other synapses on the same dendritic branch. If the total for the branch is sufficient, it can propagate deeper into the dendrite and add to the effects of other branches. If enough action potentials have recently arrived across the neuron, the neuron will fire and send out its own action potential.
CHANGES TO SYNAPTIC WEIGHTS
The electrical charge injected by an action potential encourages the target neuron to fire. The amount of charge that is injected at a synapse by one action potential is called the synaptic weight. In the case of a synapse between pyramidal neurons, the synaptic weight defines the contribution of the source receptive field to the target receptive field. In the case of a synapse made by a pyramidal neuron on to a medium spiny neuron, the synaptic weight defines the recommendation weight of the receptive field in favour of the behaviour corresponding with the medium spiny neuron. Hence changes to synaptic weights are the mechanisms implementing changes to receptive fields and to recommendation weights. These changes are the detailed mechanisms supporting memory.
The weight of a glutamatergic synapse is defined by a number of factors. One factor is the number of AMPA channels in the synapse. A related factor is the size of the synapse, because a larger synapse has room for more channels. Another factor is the amount of charge that can travle through one AMPA channel during the time it is open. Yet another factor is the proportion of AMPA channels that open, which is determined by the amount of glutamate released by the action potential. All of these factors can be influenced by different mechanisms, thus managing the synaptic weight.
When an action potential arrives at a synapse, calcium ions enter the axonic part through voltage gated calcium channels, trriggering release of glutamate. The calcium ions are removed over a period of seconds. If another action potential arrives within seconds of the first, the new calcium ion influx is reinforced by the ions remaining from the previous action potential, resulting in a largeer release of glutamate. The larger release means that a higher proportion of the AMPA receptors open in the postsynaptic density, increasing the effect of the action potential. Hence there is a temporary increase in synaptic weight that lasts for a few seconds.
After an action potential arrives at a synapse, glutamate binds to NMDA receptors and remains bound for a short while. When a pyramidal neuron fires, it launches an action potential down its axon. In addition, an action potential is launched that backpropagates into the dendritic tree of the neuron. If the backpropagating action potential arrives while glutamate is still bound to the NMDA receptor, the receptor opens and allows calcium ions to enter. An immediate result is a series of chemical reactions that increase the conductance of the AMPA receptors, in other words the amount of sodium ions they allow to pass when open. This increase decays back in a period from minutes to hours, so for that length of time the synaptic weight has increased.
If multiple action potentials arrive close together and the neuron fires, NMDA channels open and in addition sufficient glutamate diffuses to the edge of the postsynaptic density that the mGlu receptors are activated. This combination results in a cascade of chemical processes resulting in the synthesis of more AMPA channels, that are inserted in the postsynaptic density and increase the synaptic weight. However, this increase is temporary, lasting a matter of hours. The decay occurs because although the additional AMPA channels force an expansion in the size of the synapse to accommodate them, the synapse gradually reverts to its original size, squeezing out the extra channels.
If in addition to activation of NMDA and mGlu receptors, before the synapse has decayed back to its regular size a nearby dopamine synapse gets an input, a combination of chemical cascades are activated leading to synthesis of messenger RNA coding for the cytoskeleton molecule actin. This mRNA is transported to the base of the spine, where it drives a higher level of actin synthesis. The additional actin sets the synaptic size larger, resulting in room for more AMPA channels. Synaptic weight increases by this mechanism last much longer term.
MEMORY AND THE TIMESCALE OF SYNAPTIC WEGHT CHANGES
Working memory lasts for a matter of seconds. Definition of the receptive fields that support working memory depend on increased release of glutamate. Priming memory lasts for a matter of minutes, and definition of the receptive fields supporting priming memory depend on increased conductance of AMPA receptors. Semantic and episodic memory last for hours up to very long term. The shorter end of this range depend on creation of extra AMPA channels. Longer term declarative memories depend on stable increases in synaptic size. Procedural memory depends on long term changes to synaptic weights on medium spiny neurons, and also depend on stable changes to synaptic size.